Laerdal Medical silicone resuscitators and masks gain MDR approval
Laerdal Medical silicone resuscitators and masks gain MDR approval
Stavanger, Norway
30.09.2022
- Laerdal Silicone Resuscitator (LSR), Laerdal Silicone Masks, NeoNatalie Resuscitator, Upright Bag Mask, Upright Bag Mask with PEEP and Newborn Mask comply with the new EU Medical Device Regulation (MDR), reinforcing confident use by medical personnel.
- The MDR Certificate was issued by the notified body DNV Product Assurance AS after review and approval of the content of the technical file. The MDR Certificate is the ultimate evidence of compliance with the European Medical Device Regulation (MDR) and that products are of high quality. Compliance with MDR ensures continued market access to the EU, and no disruptions in the supply chain.
- Ensuring compliance with MDR further proves Laerdal Medical’s ability to develop clinically relevant ventilation solutions, strengthening its position as a trusted partner in time critical emergencies.
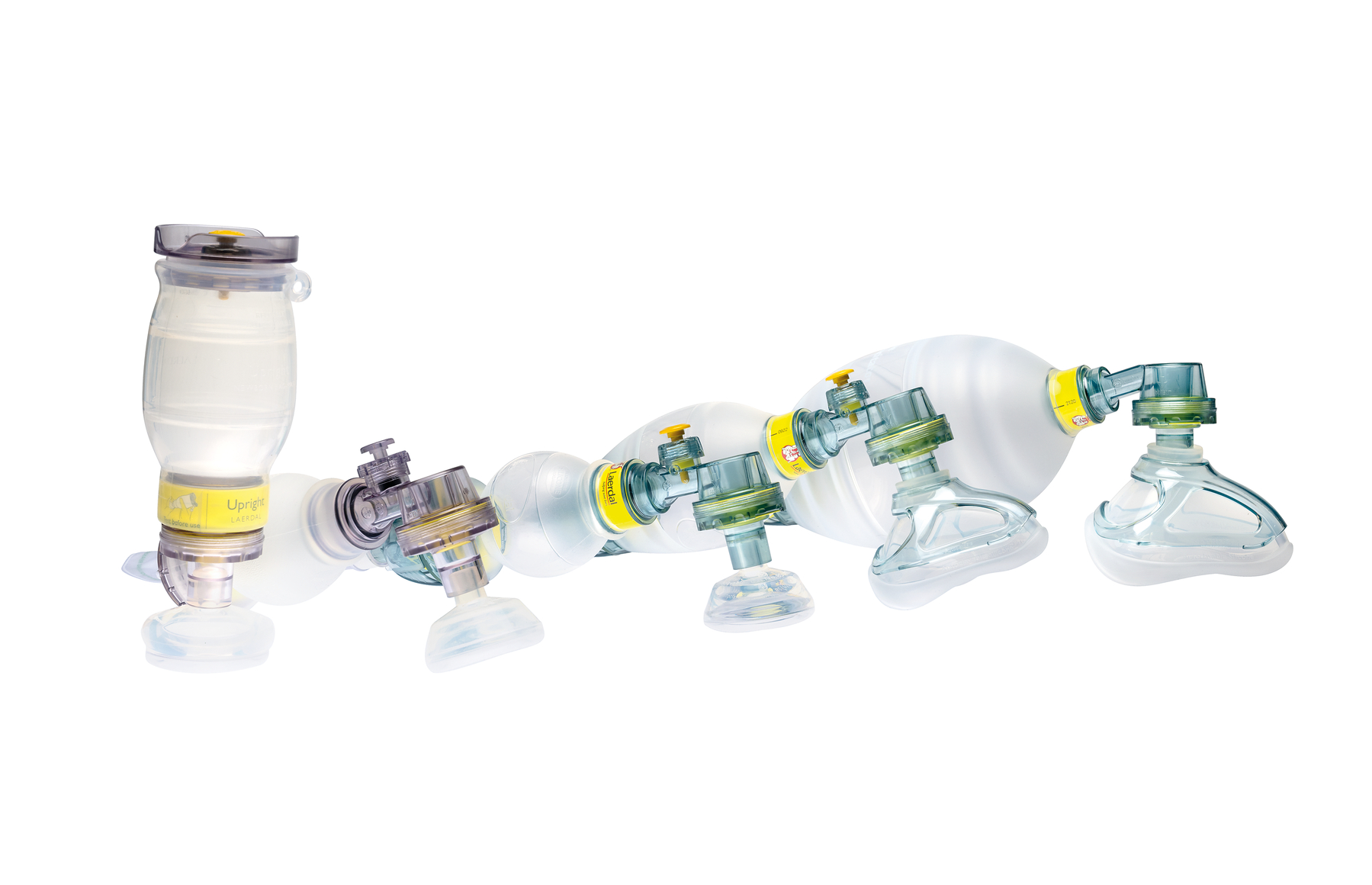
Laerdal Medical today announced that the Laerdal Silicone Resuscitator, Laerdal Silicone Masks, NeoNatalie Resuscitator, Upright Bag Mask, Upright Bag Mask with PEEP and Newborn Mask have achieved MDR certification. These reusable bag valve masks (BVM) enable medical personnel to facilitate assisted ventilation of patients in pre-hospital, in-hospital and post-hospital care. Compliance with MDR to sell and distribute medical devices in the European Union and ensures continuance in the supply of the medical devices.
The MDR certificate applies to the following products, including its accessories:
The EU MDR was published in May 2017 and became mandatory from May 2021. The EU MDR allows for a transition period that ends at the latest in May 2024. The purpose of MDR is to ensure that the medical devices are safe and effective.
Jules Eilledge, Vice President Therapy Products – Lifesaver Solutions at Laerdal Medical, said:
"Achieving this certification is a real testament to the dedication of our extended Resuscitator Product team. It means we will be able to continue to support healthcare providers throughout Europe and the rest of the world. Standards such as these ensure quality of care which very much aligns with our goal to help save one million more lives every year, by 2030."
About Laerdal Medical silicone resuscitators and masks
The Laerdal Silicone Resuscitator, Laerdal Silicone Masks, NeoNatalie Resuscitator, Upright Bag Mask, Upright Bag Mask with PEEP, and Newborn Mask are reusable bag valve masks (BVMs) and masks that are essential to safe and effective airway management during an emergency.
The Upright Bag Mask and the Upright Bag Mask with PEEP are also available as part of the Safer Births Bundle of Care through Laerdal Global Health. The scale up of Safer Births Bundle of Care in Tanzania was recently awarded $13m in funding from the World Bank for further roll out of the project.
Laerdal Global Health, a not-for-profit company of the Laerdal Group, exists to help reach the Sustainable Development Goals for maternal and newborn health (SDG3). We believe that this can be achieved if we work in close collaboration with partners to innovate and support health providers.